In the rapidly evolving healthcare landscape, the demand for evidence-driven insights has surged to the forefront. Data collection needs have evolved from set data points to a demand for continuous flows of evidence throughout an entire lifecycle of a medical product. This evolution, while enriching, has also introduced complexities for medical affairs teams.
So, what exactly is Real World Evidence (RWE) and how can it be leveraged?
RWE encompasses primary and secondary data collected through a variety of means and purposes, including claims data, survey data, and chart reviews. Going beyond a clinical trial target population, RWE captures a broader, more representative patient population that assesses the reality of what is happening in clinical practice.
Ultimately, RWE expands your evidence base. Herein lies the usefulness of RWE in both Pre- and Post-approval landscapes. RWE can help shape the contours of communication strategies and future developments across a diverse array of stakeholders including patients, caregivers, payers, and healthcare professionals.
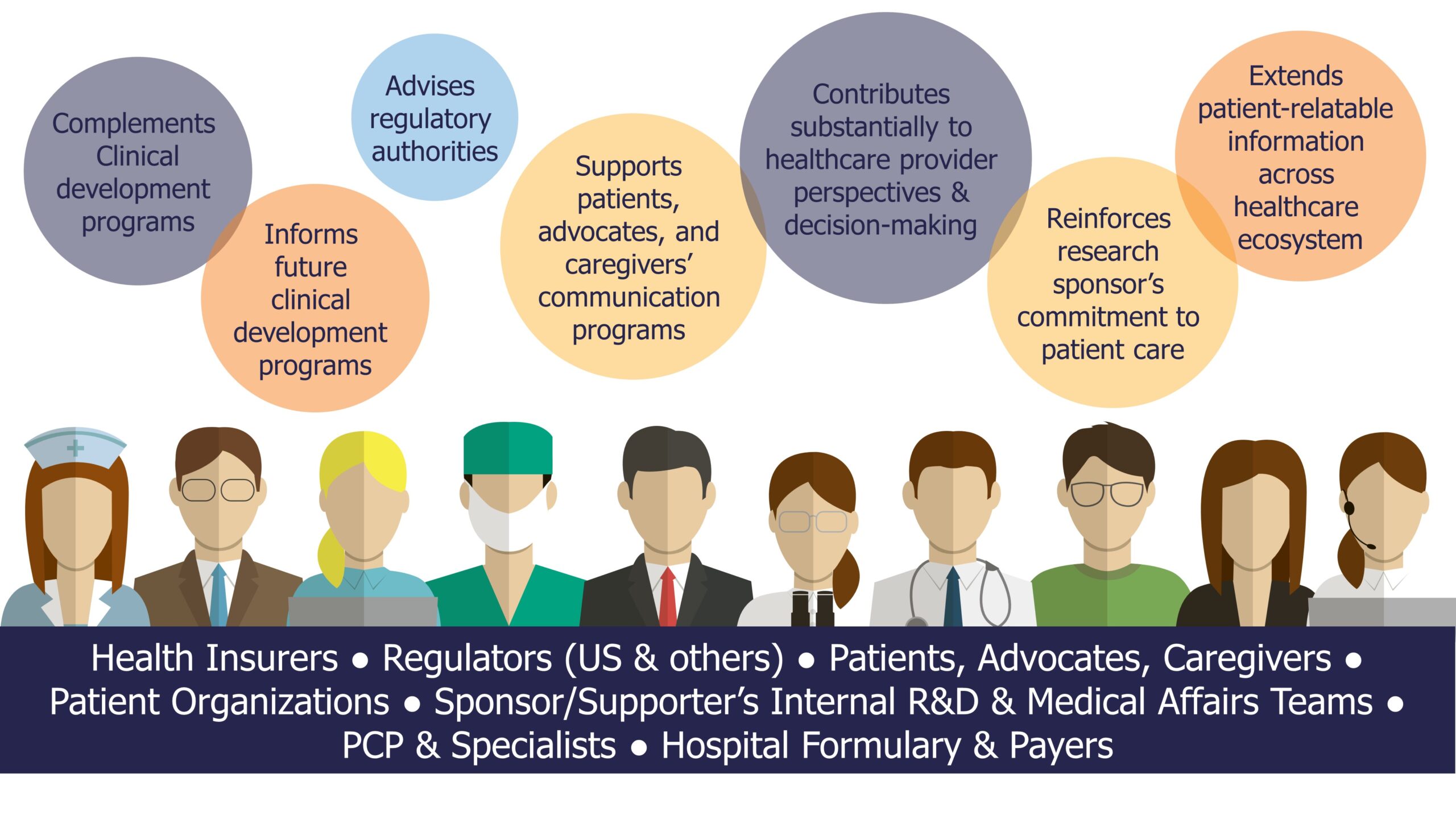
At KJT, we stand not just as observers but as architects of RWE and real-world data (RWD).
KJT’s RWE solution suite is a testament to our strong understanding of how to report research findings in an impactful way for both internal and external audiences across:
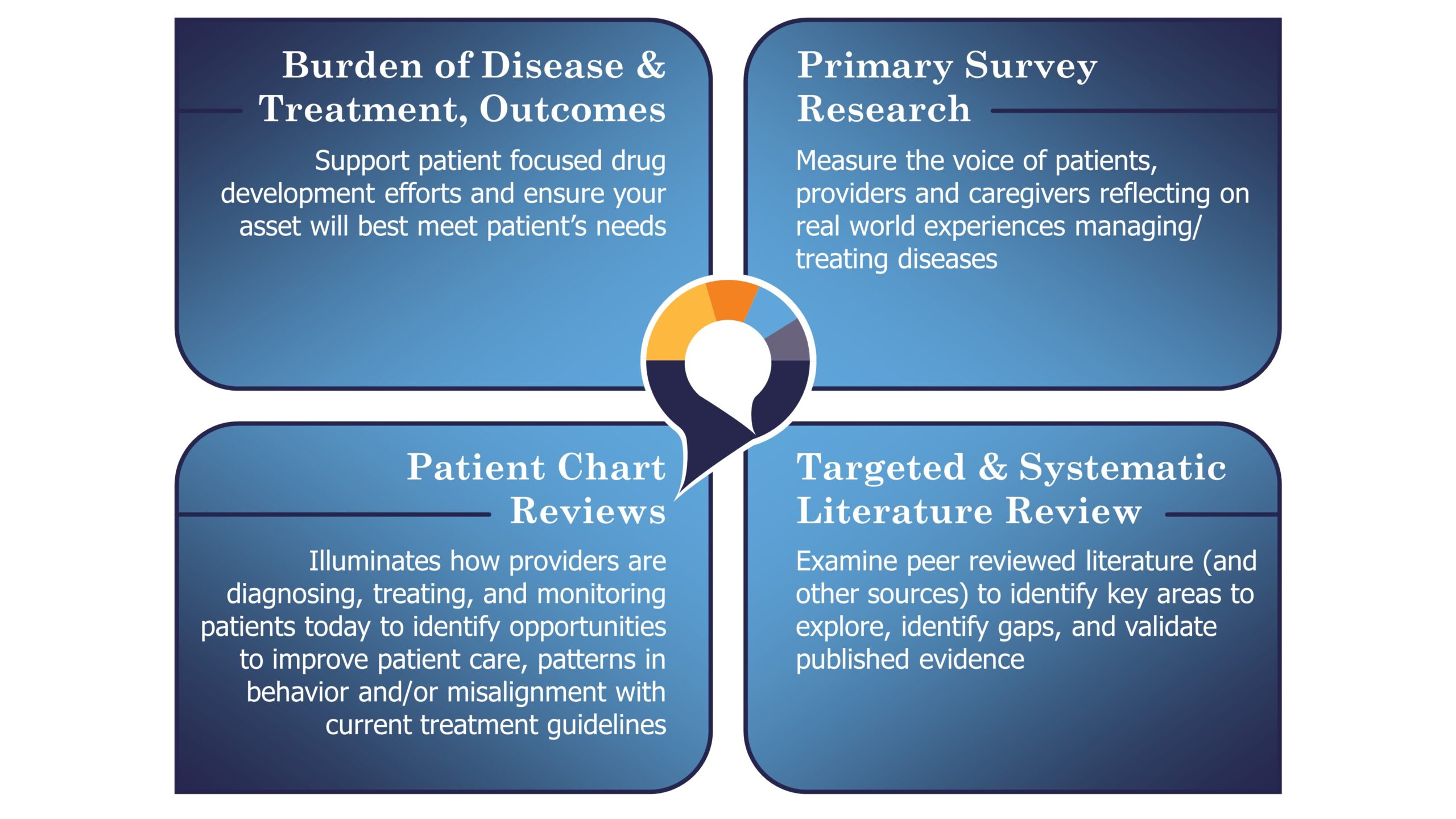
KJT’s wealth of experience managing protocol development, IRB submission, and facilitating co-creation of instrument design with KOLs and client stakeholders for primary research collection ensures every RWE engagement is valuable.
First, KJT recognizes the importance of stakeholder alignment on study objectives and methodology. To accomplish this, forging meaningful connections with key opinion leaders during the initiation of our research paves the way for delivering clear and actionable guidance.
Bolstered by the expertise of our Medical Advisory Council, KJT also possesses an intricate understanding of various therapeutic areas, a cornerstone for conducting RWE research.
In addition to ensuring adherence to timelines and quality control, our dedicated project managers understand the challenges and nuances of ethical review board submission and the importance of developing weighting schemes and precise sampling approaches to recruit clinicians and patients globally and representatively.
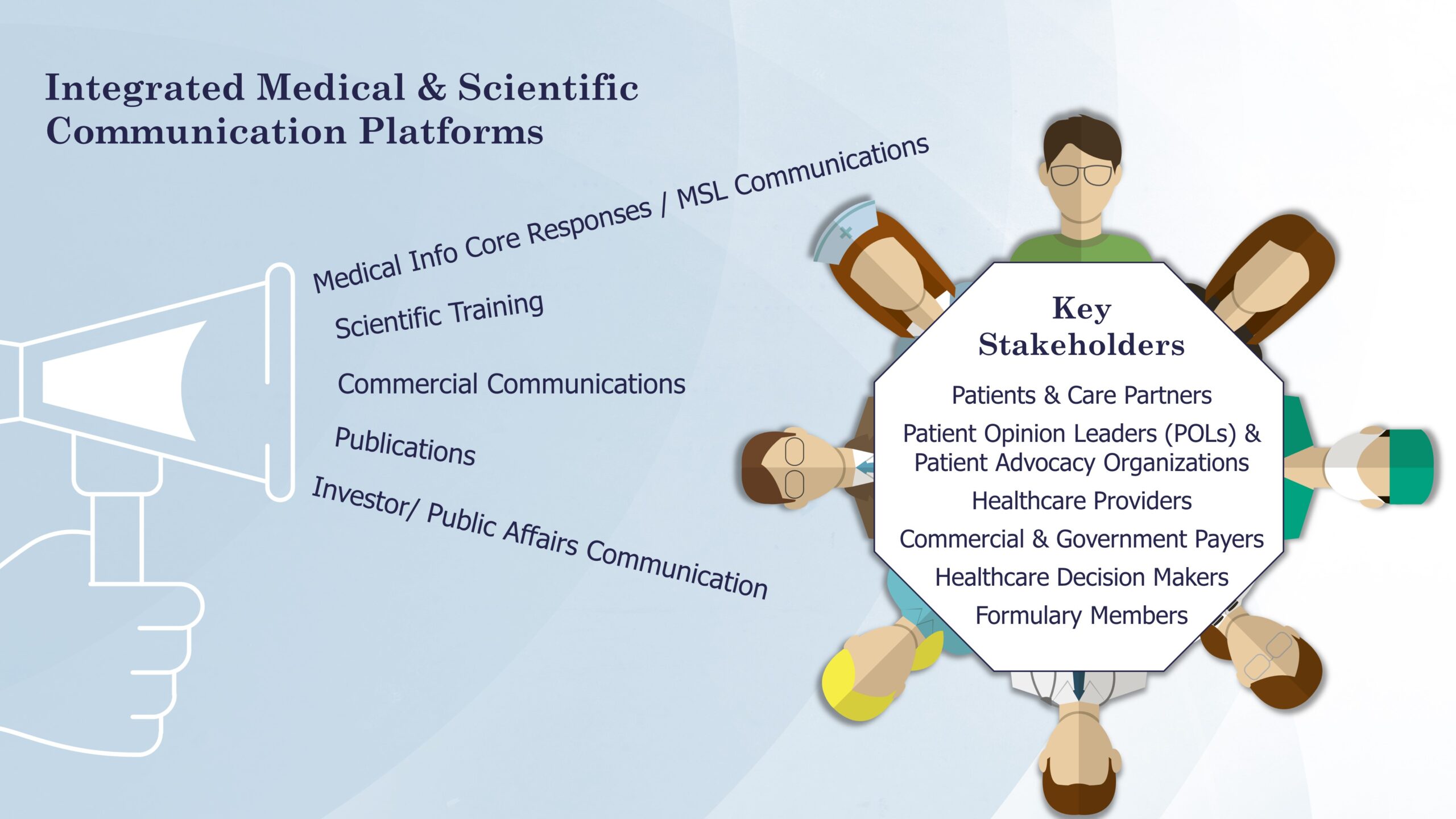
Yet, the culmination of our efforts does not conclude with the generation of insights; it extends into the art of effective communication. Our comprehensive approach incorporates numerous strategies including medical information dissemination, scientific training, commercial communications, publications, and investor and public affairs communication. These multifaceted strategies ensure that our clients’ evidence resonates deeply and decisively across the spectrum of stakeholders, imparting value at each interaction.
Real World Evidence is changing the future of healthcare delivery. With a legacy spanning over 15 years in RWE generation and communication, KJT is committed to delivering evidence-driven research and real-world application to support clients’ needs.
Author
Nick Henderson
Research Consulting Director
Nick is a skilled researcher with global healthcare market research and RWE experience in many pharmaceutical and medical device industries. As a Research Consultant, he enjoys uncovering stories in data to deliver strategic insights and evidence-based recommendations. Nick also earned his Certificate in the Principles of Market Research from UGA.
Have a question, want to learn more, or just want to say hello?
We’d love to hear from you. Fill out the form and we will be in touch.


